Automating Process Flows in SAS LSAF
By Caro Sluijter
The SAS® Life Science Analytics Framework (LSAF) renders an integrated system that provides a centralised, standardised repository and SAS programming and execution environment in a single solution. One of the key features of LSAF is the workflow capability. This capability allows you to automate processes by using process flows, to allocate tasks and to keep track of the progress of task and activities. The usage of process flows reduces the manual involvement and it automates, streamlines and tracks processes which benefits the management of your clinical research processes. A process flow is particularly useful when a process consists of several standardised tasks that need to be executed repetitively within or across studies.
This article looks at two use cases of clinical research processes with standardised tasks:
Use case #1: Exchange and validation of study data
Pharmaceutical companies collaborating with Contract Research Organisations (CROs) might exchange data via LSAF, meaning that CROs upload data and corresponding documentation to the Repository in LSAF. Once uploaded, data are checked by running validation programs or jobs and a report might be automatically created and sent to a data manager. Upon the evaluation of the report, the data manager accepts or rejects the data and notifies the CRO and project lead accordingly. Once data has been accepted, it will be copied or moved to relevant folders. This process consists of running multiple jobs or performing standardised manual tasks for every upload of data that occurs, and hence involves standardised, repetitive tasks.
Use case #2: Lifecycle of a program
A programming process consists of three phases: development, validation (or test) and production. After the programmer has finished the development of a program, the program and corresponding documentation needs to be moved to the validation folder. An independent programmer, assigned by the project manager, will perform the validation. If there are issues to be resolved after validation, the process will be moved back to the development phase for revisions. When the program has passed the validation phase, the program and corresponding documentation will be promoted to the corresponding production folders. Within this process, developers, validators and the project manager need to be notified and files move through different folders. This lifecycle consists of multiple LSAF jobs and manual tasks to be executed every time a new program is developed.
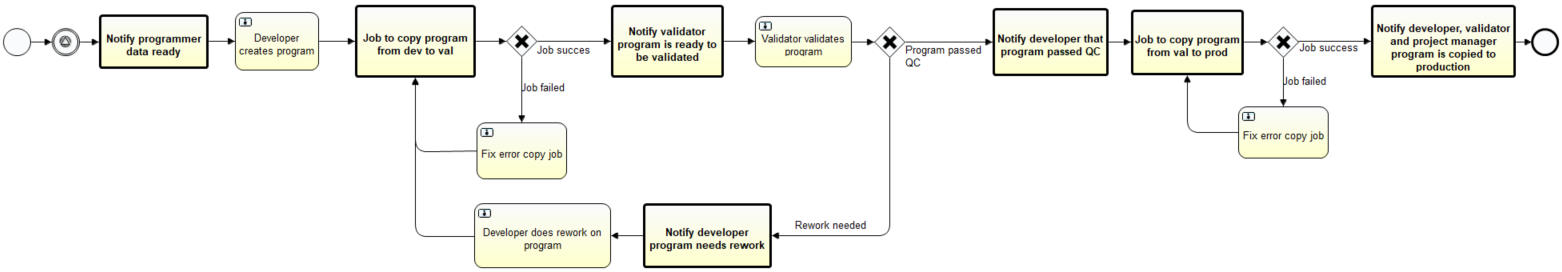
Figure 1: Example of a process flow: Lifecycle of a program
To make the life of data managers and programmers easier, OCS Life Sciences developed several process flows that expedite these processes. Figure 1 depicts an example of a process flow for the lifecycle of a program.
These process flows ensure that the next job or task is automatically started after the last one is completed, that tasks are allocated and persons assigned are being notified, and that no task or job is skipped and the correct sequence of tasks or jobs is followed. This reduces the manual participation involved, is less prone to error and it streamlines and tracks processes.
A downside to the process flows in LSAF is that for every instance the process flow is utilised, a manual set-up needs to take place. For instance, study-specific paths or study-specific notification messages need to be modified. The set-up of a process flow is a repetitive task, and as we want to eliminate repetitive tasks as much as possible, we do not want to manually set up each process flow instance. As LSAF out-of-the-box does not support autopopulation for process flow setup, we developed a solution that will automatically set up your process flow instances.
This solution is part of OCS Life Sciences’ LSAF toolkit that consultants use for the implementation of new LSAF instances or in upgrade projects. If you want to know more about OCS’ services for SAS Life Sciences Analytics Framework, get in touch with Melanie Schopp.


