Setup of ADAE and ADTTE for Exposure-Adjusted Incidence Rate Reporting
in an Integrated Summary of Safety (ISS) Submission
Abstract
Reporting Exposure-Adjusted Incidence Rate (EAIR) can be part of an Integrated Summary of Safety (ISS) submission since the regulatory agency can be interested on the drug exposure duration of the subjects until a certain adverse event (AE) occurred. The programming however, can be a challenging task, especially since the subject population in an ISS is huge and various AE categories must be reported. The adverse events analysis dataset (ADAE) can be used directly to report results in your table utilizing a macro, or alternatively a time to event analysis dataset (ADTTE) can be additionally created to facilitate easy reporting of the EAIR. This paper will talk about how to setup ADAE and ADTTE to support the EAIR analysis and why this approach is more efficient as compared to deriving EAIR at the level of table generation. Furthermore, some challenges related to integrated database development and reporting, such as huge dataset processing impacting program run times, inconsistent availability of information between individual studies resulting to more complex variable derivations and the laborious validation of analysis results against previously available reports of the individual studies, will be discussed alongside with tips and best practices to overcome these challenges effectively.
Slide 1

Slide 2

Slide 3

Slide 4

Slide 5

Slide 6

Slide 7

Slide 8
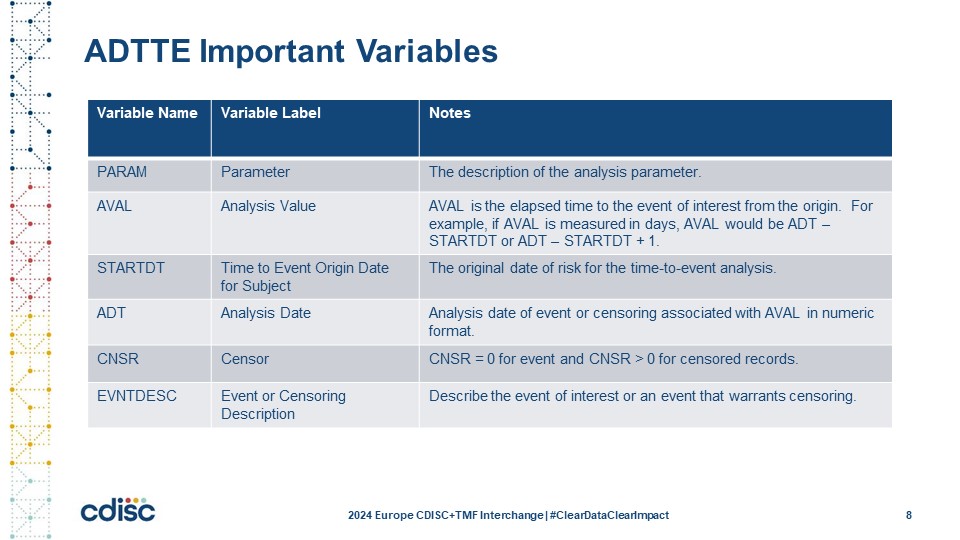
Slide 9

Slide 10
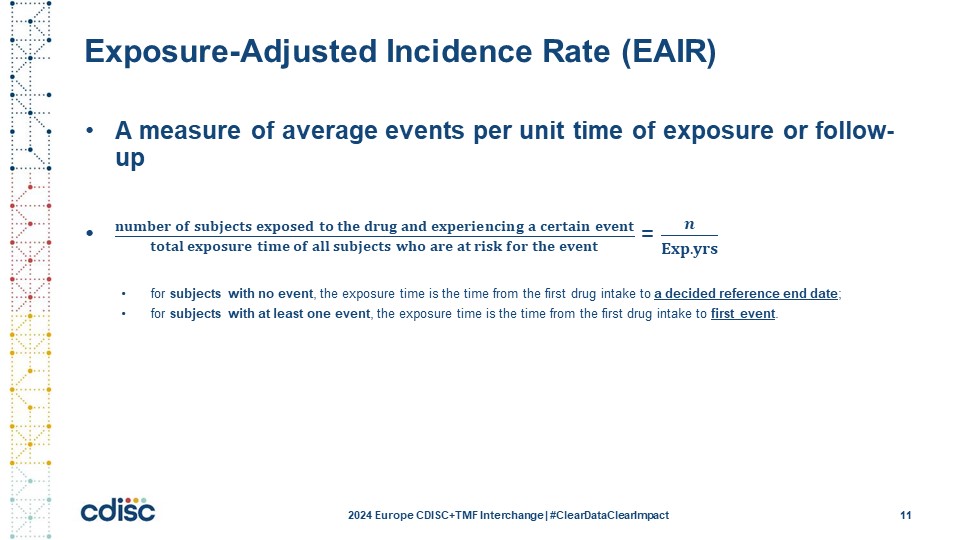
Slide 11
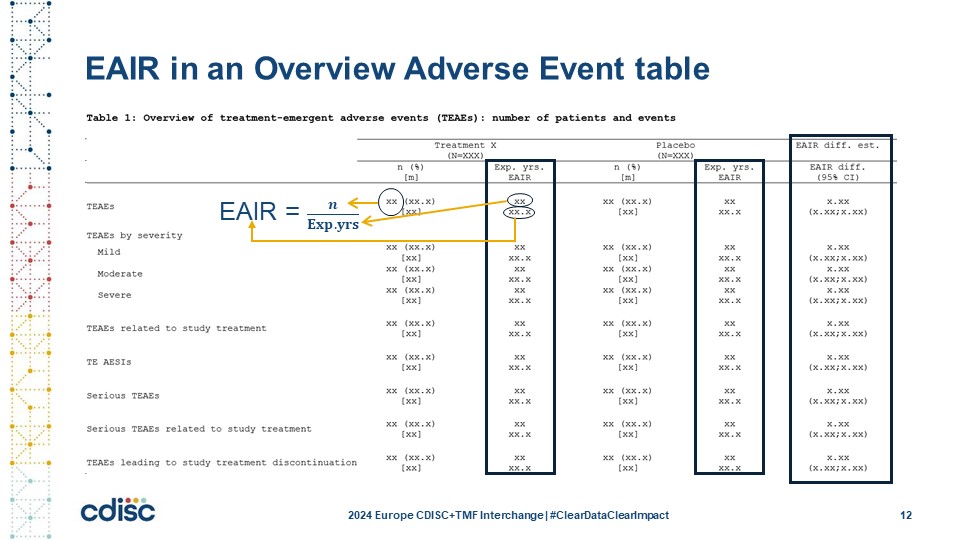
Slide 12
Slide 13
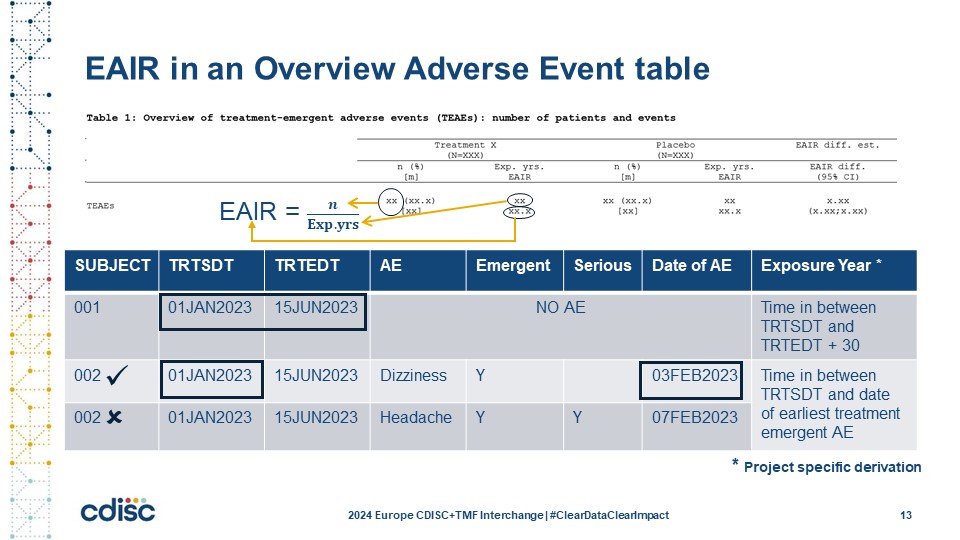
Slide 14

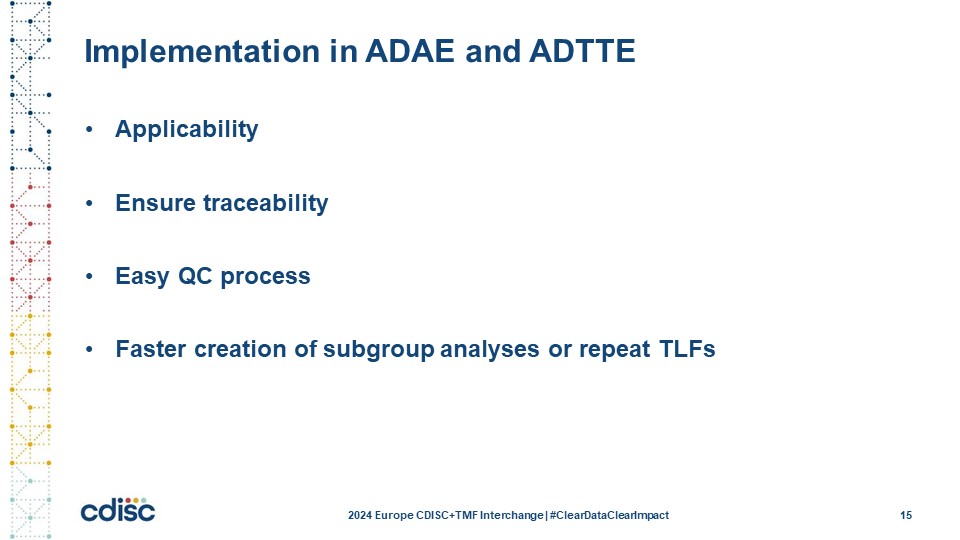
Slide 15
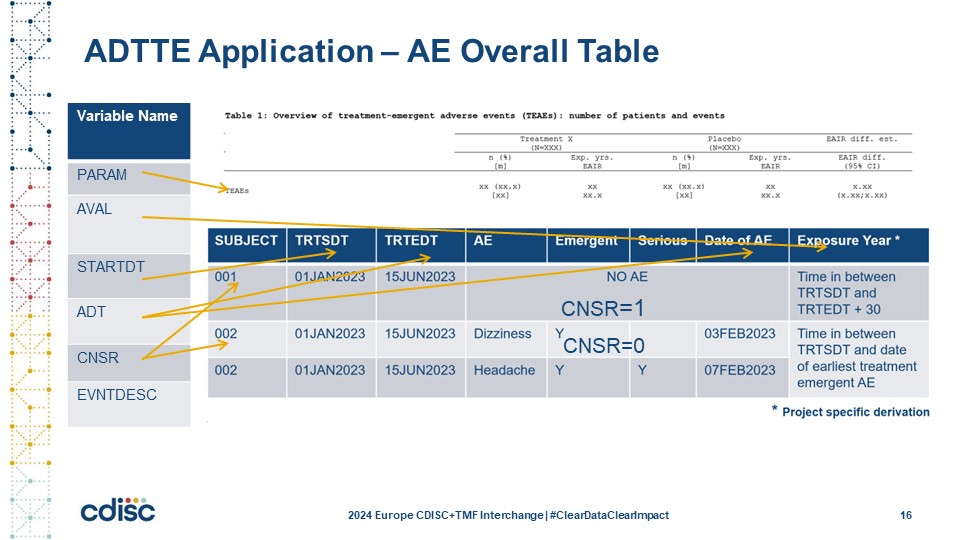
Slide 16
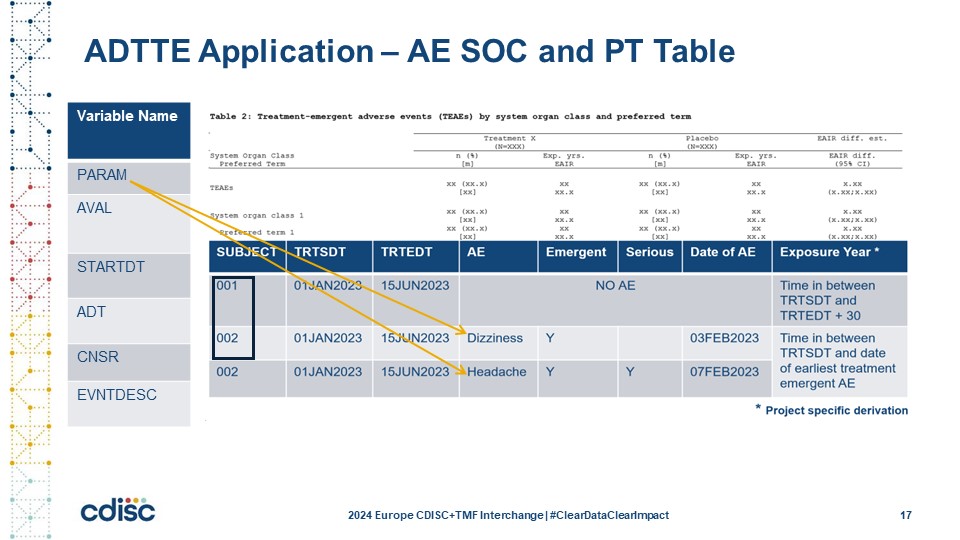
Slide 17
Slide 18
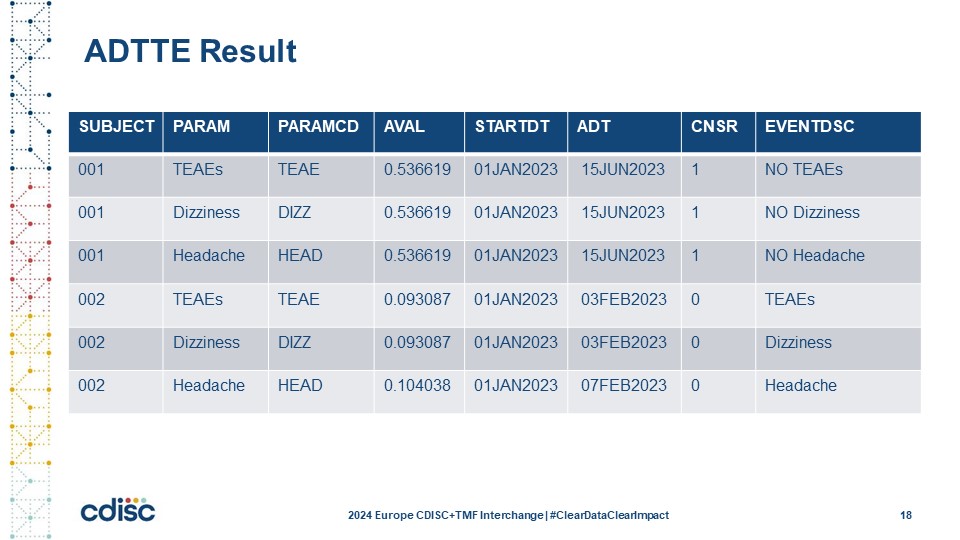
Slide 19
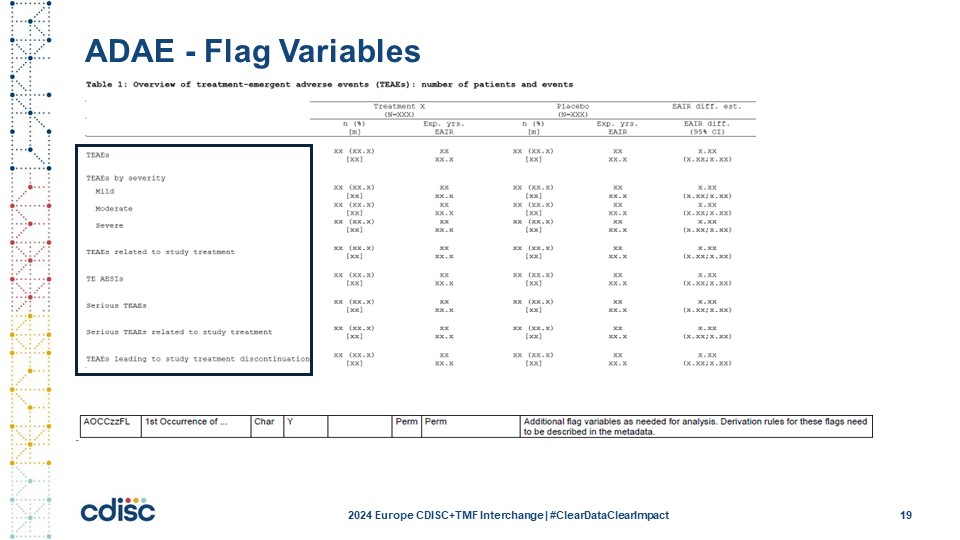
Slide 20

Slide 21
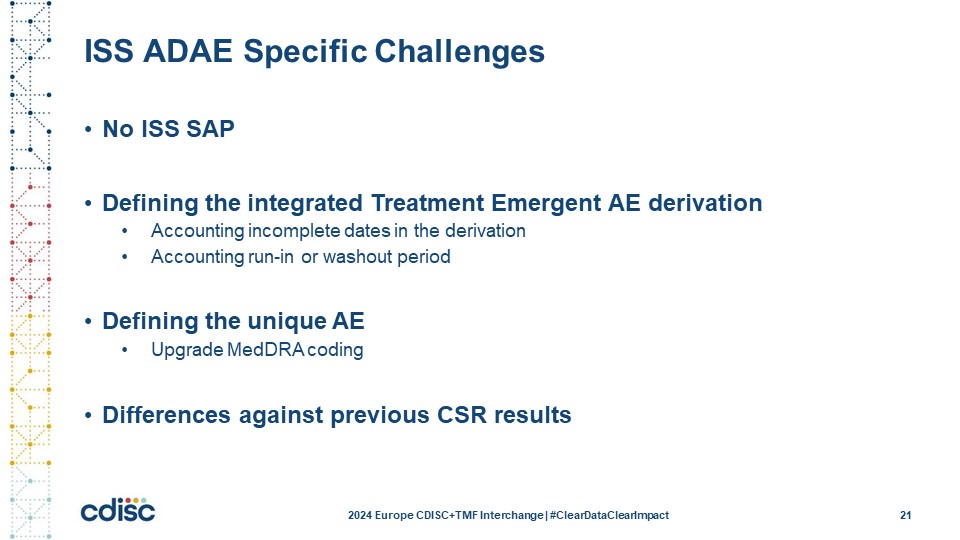
Slide 22

Slide 23

Slide 24
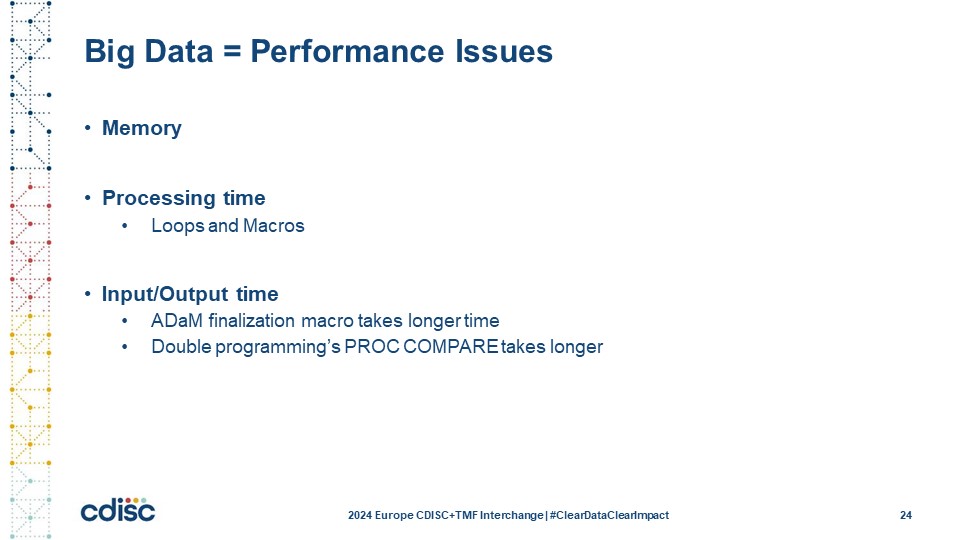
Slide 25
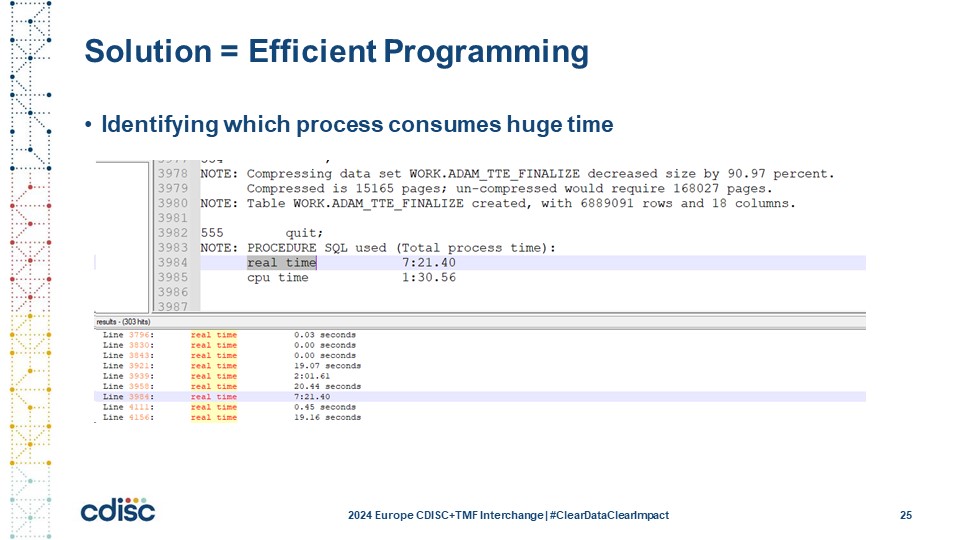
Slide 26
Slide 27

Slide 28
Slide 29
Slide 30



