Defining a SAS environment for clinical programming
SAS is the industry standard for analysing clinical data, but defining a SAS environment which meets the requirements of a life sciences company is a challenge which requires a combination of extensive industry knowledge and significant functional and technical expertise. An area in which OCS consulting has an impressive track record, for which we have recently been recognised by SAS Institute.

A recent project that OCS Consulting executed for an international pharmaceutical company was to define a new SAS environment. This article outlines some of the important considerations and an overview of the work we did for our client.
The project
Our client initiated a project to define a roadmap for the migration of their current SAS 9.4 environment to SAS Viya. The roadmap should address at least hosting, architecture, modernisation, migration impact and user experience.
Hosting
We advised our client on the different version and hosting options. Advantages and disadvantages of hosting options from On-Premises to Software as a Service (SaaS) were discussed and we supported our client in determining the appropriate sizing for compute and storage.
The overview below illustrates the differences between the hosting options. As you move further to the right an increasing number of activities are outsourced to a vendor.
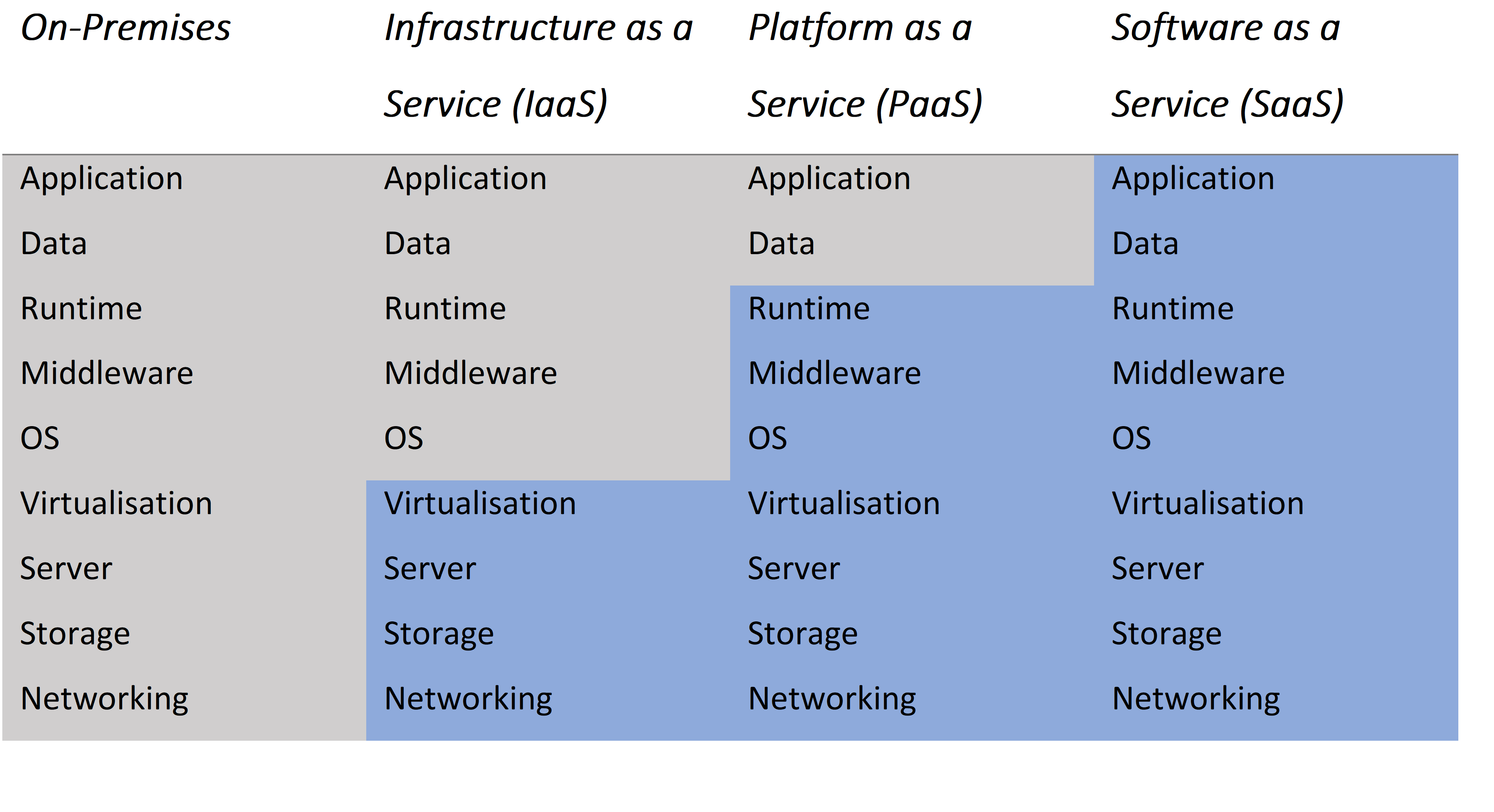
Grey = Managed by the user / Blue = Managed by the vendor
This overview helps making the high level decisions quickly. For instance, SAS Viya 4 runs on Kubernetes and if your organisation lacks the expertise to maintain this type of infrastructure, then the ‘as a service’ model becomes particularly appealing. SaaS allows users to focus entirely on their core business by outsourcing the entire technology stack. The risk of losing a critical on-staff administrator (single point of failure) is also entirely mitigated.
Architecture
A number of key factors are to be considered when designing a SAS environment. If a SaaS option is selected the vendor may take responsibility for most of these factors, however, users should still discuss at least the topics below to ensure the solution meets their requirements.
- Enterprise architecture – How does SAS fit into the organisation’s broader technical landscape? Are there enterprise-wide standards and applications which must be incorporated into the SAS architecture? Or what is the impact if they are not?
- System interfaces - No SAS environment is an island. SAS is fed data from other systems and the output of analysis may be exported. We carefully mapped all existing and (foreseeable) future dependencies and proposed methods for extracting data from various sources: the clinical data repository, Apache Hive and Medidata RAVE. SAS can access data residing in external systems through several methods such as REST API, SAS ACCESS and SAS Data Connectors. Using “proc http” SAS programs can request data from remote systems (which may not permit a direct connection through SAS ACCESS) through their REST API.
- Folder structure – Having a well-considered folder structure is imperative for the usability and maintainability of the system. We analysed the users’ current way of working and existing folder structure and incorporated the requirements and suggestions for improvement into our designs.
- Security – We discussed topics like multi-factor authentication to verify the identities of users and encryption of data at rest and encryption of data in motion to protect data.
- GDPR – We discussed topics like authorisation to restrict access to sensitive data and geographical location of the data.
- Availability – Consider disaster recovery and redundancy (replicate ‘pods’ in the case of SAS Viya) to guarantee availability in case of a failure.
- Storage – Storage should provide sufficient throughput speed and storage capacity for current and expected future use. In SAS Viya storage requires special attention as it needs to be persistent and shared across containers.
Validation of the environment was an overarching consideration and meetings with QA were conducted to ensure the environment can be qualified.
During the project our consultants spotted several opportunities for improving the performance of the current environment in terms of responsiveness of the web applications and speed of code execution and shared these findings with our client. This will directly benefit them until such time the new environment is in use.
Our combined efforts led to the creation of functional and technical designs and recommendations which were delivered to the client.
Should you also like to work with us in designing, configuring, or improving a SAS 9.4, SAS Viya or SAS LSAF environment for clinical use then please don’t hesitate to contact OCS Consulting.


