An introduction to PK and PD
by Mariska Burger and Kees Duineveld
Have you ever wondered why medication is administered in a specific form (e.g. tablet, capsule or suspension) or why certain medications are given orally and others intravenously? Have you ever wondered what happens with the medication once it has been administered or why one medication is more effective for some people than for others?
All these interesting questions can be answered by investigating the pharmacokinetics (PK) and pharmacodynamics (PD) of medication.
So, what are PK and PD? In simple terms PK is what the body does to the medication, for example, how it is absorbed into the body, distributed throughout the body to the site of action, metabolised and broken down and finally excreted from the body.
This process is referred to as ADME (absorption, distribution, metabolism, excretion). The word pharmacokinetics stems from the word kinesis, which means “movement”. PK is thus the movement of the medication into, through and out of the body.

PD on the other hand is what the medication does to the body. The word pharmacodynamics stems from the word dynamis which means “power”. The PD thus is the effect or reaction that you see (the power of the medication) in the body. For example, if you take medication for high blood pressure you expect the effect of the medication to be lowering your blood pressure.

In this article we will focus on PK. PK is influenced by many different patient related factors, such as physical attributes (gender, age, weight etc.) or a patient’s physiology (obesity, renal failure, hepatic failure etc.). These factors differ from patient to patient and influence the ADME process within a patient. These factors could explain why certain people react differently to certain medication than others or why certain people have to take higher or lower doses of a certain medication than others. PK is also influenced by the drug’s chemical properties. These properties are determined by the drug’s molecular structure, so in practice considered fixed.
PK plays a huge role in the development process of a new medication. PK is used to determine an initial safe dose to administer, to determine how often the medication should be taken and which formulation (e.g. tablet or capsule) is the best. PK is also used to proof that a generic medication is “equivalent” to the medication already on the market or to see differences between for example healthy subjects and patients with renal or hepatic impairment. Some PK parameters are also used in diagnosing impaired glucose tolerance (IGT) in diabetic patients or even to calculate the Glycaemic Index (GI) for food in nutrition.
The concentration levels of the medication are measured in the blood plasma and also in the urine of patients and usually measured over a period of 48 to 72 hours, depending on the properties of the medication. The common analysis in clinical trials is non-compartmental analysis (NCA) where no model is chosen for concentration as function of time. In NCA the two most important PK variables measured in the blood plasma are the rate of absorption (expressed by the variable Cmax – the maximum concentration level) and the extent of absorption (expressed by the variable AUC – the area under the concentration-time curve). The most important urine PK variables are the cumulative amount of drug excreted over time (Ae) and the renal clearance (CLR).
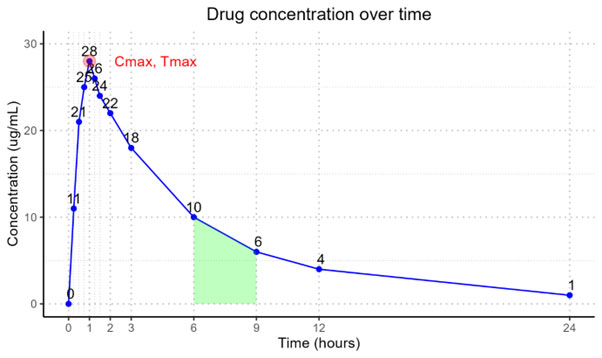
If we take for example an orally administered dose, the concentration profile over time will look something like in the figure below. You will have an absorption phase from time 0 hours to 1 hour where the absorption of the medication is greater than the elimination and the elimination phase from 1 hour to 24 hours where the elimination of the medication is greater than the absorption. The maximum concentration (Cmax) is 28 ug/mL and it is reached after 1 hour in this example, which is referred to as the time to maximum concentration (tmax). The total area under the concentration-time curve (AUC) is often calculated using the linear trapezoidal method, although other methods also exist, such as log-linear trapezoidal, Lagrange polynomial integration and Purves methods. With the linear trapezoidal method AUC is simply calculated by connecting each adjacent concentration with a straight line, calculating the area of each “trapezoid” (see shaded area in the figure below for an example of one trapezoid) between adjacent timepoints and finally summing all areas over the total sampling time (24 hours in the figure below).
Have you ever wondered how long does it take until there is no more medication left in your body? This can easily be answered by another very important plasma PK variable called the half-life (t½) of the medication. Simply put it is the time it takes for half of the administered dose to be metabolized and eliminated from your body. If you have the half-life of a medication you can easily calculate how long it will take to be completely excreted from your body. After 5 half-lives 96.88% of the medication will have been eliminated from your body. This information is also important to determine how often medication, for example antibiotics, should be administered in order for the drug to reach an effective level.
Understanding the PK and PD of medication plays a crucial role in the development of new medication. At OCS Life Sciences we are proud to say that we have a small dedicated PK team who are enthusiastic about calculating PK variables and we use industry standard Phoenix WinNonlin software to do so. If you would like to know more about PK and see who OCS can support you please get in touch with Erik Hamminga at erik.hamminga@ocs-consulting.com.


